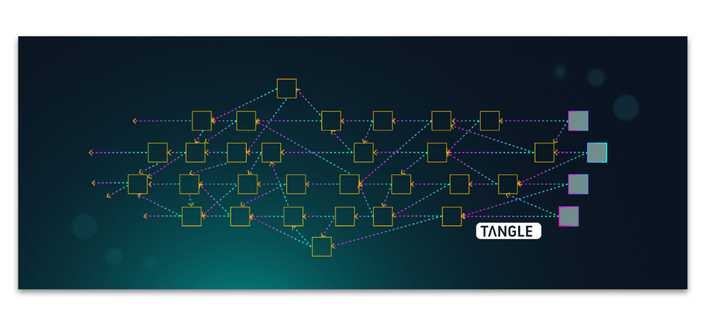
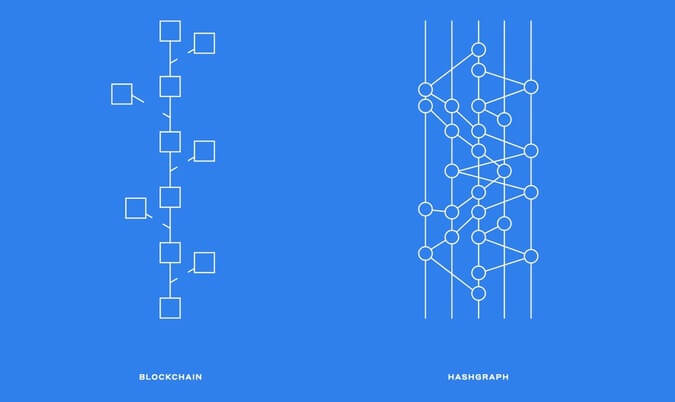
Bitcoin is the first known example of blockchain, however blockchain goes well beyond the realms of bitcoin and cryptocurrency use cases. One of the earliest and currently predominating DAG DLT platforms is IOTA which has proved itself in a plethora of use cases that go well beyond what blockchain could currently achieve, particularly within the realm of the internet of things (IOT). In fact Iota has been developing an industry data marketplace active since 2017 which makes it possible to store, sell via micro-transactions and access data streams via web browser. For the purposes of this article we will focus on DLT applications in general and include use-cases in which blockchain or DAGs can be employed interchangeably. Before we begin, what is Distributed Ledger technology?
In this sense DLT is able to speed up transactions and processes, while reducing cost, due to the removal of a middle-man or central authority overseeing each transaction, or transfer of information. DLT can be public or private in nature. A private blockchain, for example, does have trusted intermediary who decides who is to have access to the blockchain, who can participate on the network, which data can be viewed by which participants. In the context of clinical and life sciences research this could be a consortium of interested parties, ie the research team, or an industry regulator or governing body. In a private blockchain, the transactions themselves remain decentralised, while the blockchain itself has built in permission layers that allow full or partial visibility of data depending upon the stakeholder. This is necessary in the context of sharing anonymised patient data and blinding in randomised controlled trials.
In a clinical trials context the job of the data monitoring committee, and any other form of auditing becomes much more straight forward. DLT also allow for complete transparency in all financial transactions associated with the research. Funding bodies can see exactly where all funds are being allocated and at what time points. In-fact every aspect of the research supply-chain, from inventory to event tracking, can be made transparent to the desired entities. Smart contracts operate among participants in the blockchain and also between the trusted intermediary and the DLT developer whose services have been contracted for building the platform framework, such as the private blockchain. The services contracts will need to be negotiated in advance so that the platform is tailored to adequately conform to individualised study needs. Once processes are in place and streamlines the platform can be replicated in comparable future studies.
DLT can address the problem of duplicate records in study data or patient records, make longitudinal data collection more consistent and reliable across multiple life cycles. Many disparate stakeholders, from doctor to insurer or researcher, can share in the same patient data source while maintaining patient privacy and improving data security. Patients can retain access to the data and decide with whom to share it with, which clinical studies to participate in and when to give or withdraw consent.
DLT, such as blockchain or DAGs, can improve collaboration by making the sharing of technical knowledge easier and centralising data or medical records, in the sense that they are located on the same platform as every other transaction taking place. This results in easier shared access by key stakeholders, shortening of negotiation cycles due to improved coordination and making established clinical research processes more consistent and replicable.
From a statisticians perspective, DLT should result in data of higher integrity which yields statistical analysis of greater accuracy and produces research with more reliable results that can be better replicated and validated in future research. Clinical studies will be streamlined due to the removal of much bureaucracy and therefore more time and cost effective to implement as a whole. This is particularly important in a micro-environment with many moving parts and disparate stakeholders such as the clinical trials landscape.
References and further reading:
From Clinical Trials to Highly Trustable Clinical Trials: Blockchain in Clinical Trials, a Game Changer for Improving Transparency?
https://www.frontiersin.org/articles/10.3389/fbloc.2019.00023/full#h4
Clinical Trials of Blockchain
Blockchain technology for improving clinical research quality
https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-017-2035-z
Blockchain to Blockchains in Life Sciences and Health Care
https://www2.deloitte.com/content/dam/Deloitte/us/Documents/life-sciences-health-care/us-lshc-tech-trends2-blockchain.pdf


great article, very informative. I’m wondering why more
experts of this sector don’t notice this. You should continue your writing.
I am sure, you have a great readers’ base already!