The development of new drugs starts far before they are even seen in clinical trials. The discovery of multiple candidate drugs occur early on in the development process, often as a result of new information about how a disease functions, large-scale screening of small molecules, or the release of a new technology.
After a promising drug has been found, pre-clinical studies can be performed. A pre-clinical study for a new drug is used to determine important information about toxicity and suitable dosage amounts. These studies can be in vitro (in cell culture) and/or in vivo (in animal models) and determine whether a treatment will continue to the clinical trials stage.
Clinical trials test whether these experimental treatments are safe for use in humans, and whether they are more effective in treating or preventing a disease when compared to existing treatments. Clinical trials consist of several stages, called phases, where each phase is focused on answering a different clinical question: Progression of a treatment to the next phase requires the study to meet several parameters to ensure a treatment’s safety or efficacy.
- Phase 0: Is the new treatment safe to use in humans in small doses?
- Phase I: Is the new treatment safe to use in humans in therapeutic doses?
- Phase II: Is the new treatment effective in humans?
- Phase III: Is the new treatment more effective than existing treatments?
- Phase IV: Does the new treatment remain safe and effective post-market?
Phase 0: Small dose safety
Phase 0 studies can help to streamline the other clinical trial phases. Phase 0 consists of giving a few patients small, sub-therapeutic doses of the new treatment. This is to make sure that the new treatment behaves as expected by researchers and isn’t harmful to humans prior to using higher doses in phase I trials.
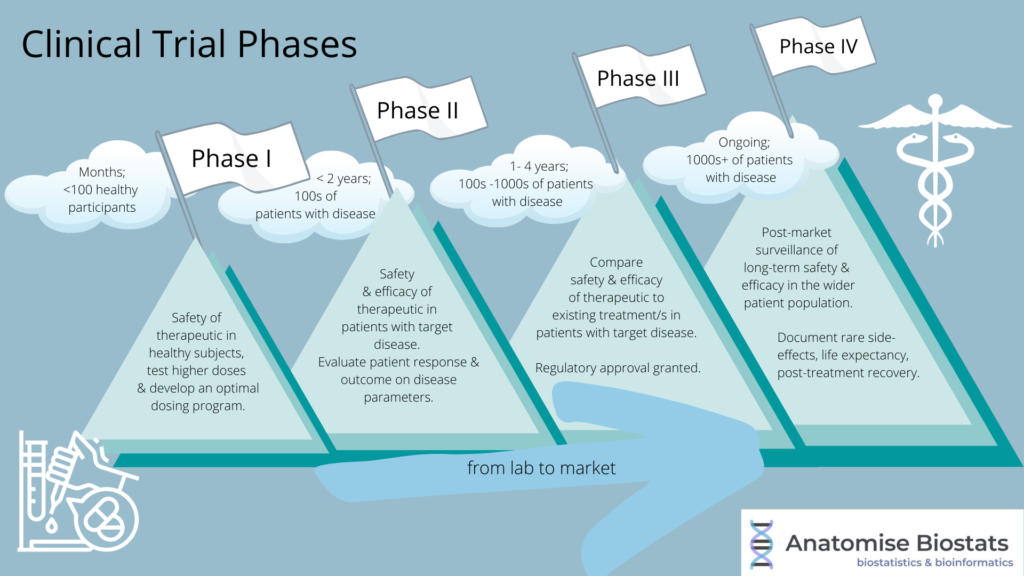
Phase I: Therapeutic dose safety
Phase I studies evaluate the safety of various doses of the new treatment in humans. This takes several months with typically around 20-80 healthy volunteers. In some cases, such as in anti-cancer drug trials, the study participants are patients with the targeted cancer type. A treatment may not pass phase I if the treatment leads to any serious adverse events.
Initial dosages in phase I studies can be informed based on data obtained during pre-clinical animal studies, and adjustments can be made to investigate the treatment’s side effect profile and develop an optimal dosing program. This could also include comparing different methods of giving a drug to patients (e.g., oral, intravenous etc.).
Phase II: Treatment efficacy
After passing phase I trials and having proven safety in humans, a new treatment advances to phase II studies designed to assess whether it may prevent or treat a disease. This phase can take between several months to 2 years, testing the new treatment in up to several hundred patients with the disease. Using a larger number of patients over a longer time period provides researchers with additional safety and effectiveness data, which is essential for the design of phase III trials.
To further test safety and efficacy, it is common to have a control group that receives either a placebo (a harmless pill or injection without the new treatment) or other current treatment (in trials where the disease is fatal unless treated e.g., cancer).
Phase III: Comparing to current treatments
Phase III studies are the last stage of a clinical trial before a new treatment can be approved for market use. The primary focus of a phase III study is to compare the safety and efficacy of a new treatment with current, existing treatments in patients with the target disease. Anywhere from several hundred to 3,000 patients may be included in a phase III study for between 1 to 4 years. Due to the scale of this phase, long-term or rare side effects are more likely to be uncovered.
Phase III studies are often randomised control trials, where patients will be randomly designated to different treatment groups. These groups may receive placebo, a current treatment (control group), the new treatment, or variations of the new treatment (e.g., different drug combinations). Randomised control trials are often double-blinded, where both the patient and the clinician administering their treatment do not know which treatment group they are assigned to.
A new treatment may continue to market and phase IV trials if the results prove it is as safe and effective as an existing treatment.
Phase IV: Post-market surveillance
If a new treatment passes phase III and is approved by the MHRA, FDA, or other national regulatory agency, it can be put to market. Phase IV is carried out in the post-market surveillance of the new treatment to keep updated on any emerging or long-term safety and efficacy concerns. This may include rare or long-term adverse side effects that were not yet discovered, or long-term analyses to see if the new treatment improves the life expectancy of a patient after recovery from disease.
Summary
Clinical trials are ultimately designed to mitigate risk. This includes the risk to the safety of trial participants by limiting the use of potentially unsafe treatments to small doses in a small number of patients before scaling up to testing therapeutic dose safety. Risk mitigation is not only for patient safety but also for preventing financial misspending as a treatment that is deemed unsafe in phase 0 would not proceed to the later, more costly clinical trial phases.
Not all clinical trials are the same, however, as each trial will have a different disease and treatment context. Trials for medical devices are somewhat different from pharmaceutical trials (for more information about the differences between medical device and pharma trials, click here). In addition, while sample sizes expand with phase progression, the required sample size for each trial and each phase is dependent on several factors including disease context (a rare disease may require lower sample sizes), patient availability (location of trial), trial budget and effect size. The sample size values mentioned earlier in this blog are purely indications of what each phase may use (for more information on how a biostatistician determines a suitable sample size, click here).
References
https://www.fda.gov/patients/drug-development-process/step-3-clinical-research
https://www.healthline.com/health/clinical-trial-phases
