
BIOSTATISTICS SERVICES
Biostatistics expertise for every stage of your R&D and clinical trials.
Biostatistics is about making your clinical trial data work for you. We design studies to best address the questions that matter most. Whether it’s calculating sample sizes that maximise resources, creating randomisation plans to reduce bias, or efficient nuanced analysis that delivers clear, actionable results – biostatistics can make all the difference to your study outcomes. Our approach is rooted in understanding your specific goals and providing a solid statistical foundation, so your study outcomes are not only reliable but also meaningful for decision making and regulatory review. By focusing on what’s essential, we help you get the insights you need to move forward with confidence.

Biostatistics help for R&D or exploratory studies
At the exploratory stage, biostatistics helps medtech companies gather essential data on a device’s feasibility, safety, and functionality. Early studies often focus on key areas such as performance, proof of concept, and safety indicators, using comparative analyses and optimised device parameters to fine-tune the technology before clinical trials. By providing a structured, data-driven approach, these studies offer clear, actionable insights that help teams make informed decisions about moving forward. With this foundation, you can reduce risk, streamline development, and prepare confidently for the next stage of clinical testing.
Biostatistical design & analysis of medtech clinical trials
Up-to-date biostatistical methods and clinical study design techniques ensure that your research is as innovative as your therapeutic. Assistance in the development of a statistical analysis plan for your trial, including sample size calculations, adjustments for missing data, and establishing early-stopping conditions are just some of the ways our biostatistical expertise could add value to your project.
Some medtech trials benefit from adaptive designs that allow flexibility as new data emerges. When appropriate, adaptive methods like Bayesian analysis enable adjustments to device parameters, patient criteria, or endpoints, supporting options like early stopping and sample size recalibration. Thoughtfully integrated, adaptive designs ensure your trial remains efficient and aligned with regulatory and clinical expectations.
Our well-considered approach will help to determine the best way forward in achieving your specific clinical study goals subject to the data and resources available.
BIOSTATISTICS SERVICES IN DETAIL
- Comprehensive Clinical Study Design, Statistical Analysis and Reporting
- Bayesian Techniques & Clinical Trial Design
- Bayesian Adaptive Designs
- Clinical Survey Design & Analysis
- Statistical Programming
Our biostatistical consulting services are individually tailored to suit the project at hand and can cover a broad range of approaches. We can theoretically enter into collaboration at any stage of your research, whether it be the design stage, data collection, statistical analysis, reporting and preparing for presentations. Most commonly a statistical consulting project will cover one or more of the following areas:
Biostatistics Services for Study Design and Planning in Clinical Trials
Study design and planning are critical elements for the success and validity of clinical trials. Our biostatistical consulting services offer tailored solutions to ensure the scientific rigour and reliability of your research. Here are some key aspects of our biostatistics services involved in study design and planning for clinical trials:
Study Design and Protocol Development:
We collaborate closely with your research team to determine the most appropriate study design based on your research objectives, available resources, and ethical considerations. Our experts assist in defining the study population, eligibility criteria, endpoints, and estimands which form essential components of the study protocol.
Sample Size Calculation:
Accurate sample size calculation is vital for obtaining statistically significant results. Our biostatisticians utilise statistical methods to calculate the optimal sample size, considering factors like expected effect size, variability, significance level, and statistical power. This ensures that your trial has sufficient participant numbers to detect clinically meaningful differences.
Randomisation Schedules:
To reduce bias and ensure comparability between groups, randomisation is employed to allocate participants randomly to different treatment groups. We design randomisation schedules or algorithms, using techniques like simple randomisation, stratified randomisation, or block randomisation.
Statistical Analysis Plan (SAP):
The SAP is a comprehensive document that outlines the statistical methods to be used in analysing the collected data. Working closely with your research team, we develop the SAP, which includes descriptions of primary and secondary endpoints, analysis populations, statistical tests, and handling of missing data. The SAP guides the data analysis process, promoting transparency and consistency.
CDISC Compliant SDTM and ADaM Data Set Preparation:
Ensuring compliance with Clinical Data Interchange Standards Consortium (CDISC) guidelines, we prepare your clinical trial data in standardised formats, such as the Study Data Tabulation Model (SDTM) and the Analysis Data Model (ADaM). This conversion of raw data enhances data integration and facilitates regulatory submissions.
TLFs (Tables, Listings, and Figures) Mock Shells:
TLFs are essential components of clinical trial reporting, presenting key findings in a clear and concise manner. Our biostatisticians create mock shells for TLFs, outlining the layout and structure of tables, listings, and figures that will be generated during data analysis. These mock shells ensure consistency in reporting and enhance data visualisation.
Meta-Analysis and Bayesian Simulation Techniques using MCMC for Clinical Trial Planning
Meta-Analysis and Bayesian simulation techniques are valuable tools that can aid in the planning of clinical trials, providing insightful information for decision-making and study design optimization.
Meta-Analysis:
Meta-analysis involves the systematic review and quantitative synthesis of data from multiple clinical studies. It allows researchers and biostatisticians to pool information from various trials, providing a comprehensive and more precise estimate of treatment effects or associations. Meta-analysis is particularly useful when individual studies might have insufficient statistical power to detect small effects. By combining data from multiple trials, researchers can obtain more robust conclusions, informing the planning of new trials by identifying gaps in existing evidence or determining the most promising interventions to investigate further.
Bayesian Simulation Techniques using MCMC (Markov Chain Monte Carlo):
Bayesian methods are increasingly used in clinical trial planning due to their ability to incorporate prior information, update knowledge as data accumulates, and quantify uncertainty more intuitively. Bayesian simulation techniques, such as Markov Chain Monte Carlo (MCMC), provide a flexible approach to assess various design scenarios and their impact on trial outcomes. By simulating data under different assumptions and incorporating prior distributions, researchers can explore the potential influence of various factors on the study's statistical power and expected outcomes. This allows for informed decision-making and optimization of the study design before conducting the actual trial.
Our biostatistics services in clinical trial study design and planning are designed to cater to your specific needs, ensuring that your research yields robust and scientifically sound results. incorporating meta-analysis and Bayesian simulation techniques can enhance the planning of clinical trials by leveraging existing evidence and simulating different scenarios. We collaborate closely with your team to provide solutions that advance medical knowledge and improve patient outcomes. Contact us today to discuss how our expertise can benefit your clinical trials.
Statistical Analysis of Study Data and Statistical Analysis Report (SAR) for Clinical Trial Data
A well-defined Statistical Analysis Plan (SAP) guides the statistical analysis of clinical trial data. Following the SAP, the Statistical Analysis Report (SAR) becomes a crucial deliverable that summarizes the findings and conclusions of the study.
1. Statistical Analysis of Clinical Trial Data:
Upon completion of data collection, biostatisticians analyse the clinical trial data according to the pre-specified SAP. This process includes data cleaning, exploratory data analysis, and application of appropriate statistical methods to address the research questions and objectives. Biostatisticians perform inferential analyses, such as hypothesis testing and estimation of treatment effects, to draw meaningful conclusions from the data.
2. Statistical Analysis Report (SAR) as a Deliverable:
The SAR serves as a comprehensive documentation of the statistical analyses conducted in the clinical trial. It includes detailed descriptions of the statistical methods applied, any adjustments for multiple comparisons or potential confounding factors, as well as the results of all analyses performed. The SAR should be transparent and replicable, allowing regulatory agencies and other stakeholders to assess the validity of the study's findings.
3. Interim Analyses in Clinical Trials:
Interim analyses are an essential feature of some SAPs, particularly in long-duration trials or when ethical considerations necessitate early assessment of treatment effects. Biostatisticians conduct interim analyses at predetermined time points, using appropriate statistical methods to evaluate the accumulating data while preserving the overall trial integrity. Interim analyses can inform early termination, sample size adjustments, or modifications to the trial protocol, ensuring efficient use of resources and reducing participant burden.
During the analysis phase, adherence to the SAP and the subsequent generation of a comprehensive SAR ensures the validity and transparency of study results. Planned interim analyses provide an opportunity for data-driven decisions, optimising trial conduct and maximising the potential impact of clinical research.
Bayesian meta-analysis
Meta-analysis or evidence synthesis is a necessary part of clinical trial design which serves to optimise the insights and accuracy of the present trial. This is achieved by combining evidence from previous trials. Accurately combining evidence from multiple sources requires a hierarchical framework. This can be more reliably achieved by use of Bayesian rather-than frequentist meta-analysis methods. Bayesian meta-analysis is beneficial on a stand-alone basis as well as in the planning of any clinical trial and is an integral initial planning stage of any adaptive clinical trial design.
Bayesian adaptive designs for phase 1-3 clinical trials
Adaptive clinical trial design entails the prospective planning and detailed specification of methodological adaptation to be implemented in clinical trials in cases where the need arises due to changes in trial environment and updates in knowledge such as drug effectiveness data. As well as avoiding clinical trial failure by managing uncertainty and optimising resources, thereby increasing time and cost efficiency, as well as the flexibility to end an unproductive trial early or update treatment doses without running subsequent dose-finding trials.
The initial protocol stage of a phase one trial is the most ideal stage to incorporate the adaptive design, the use of adaptive design can however be initiated at any phase of clinical trial provided the data for that phase remains blinded to all parties. Adaptive trial design can be of benefit within any individual phase of clinical trial as well as highly beneficial in optimizing the entire clinical research process between phases 1 to 3 of any clinical trial sequence.
Bayesian predictive models/ Bayesian generative models
Statistical models are a simplification of reality that aim to capture primary factors underlying a system under investigation based on observed data. The aim of model fitting is to increase understanding of specific factors making up an underlying system. Statistical models can vary from quite simplistic to increasingly complex as the situation necessitates. Model fitting involves estimating model parameters in line with observed data.
Bayesian model development consists of:
- Determining a prior distribution based on previous data and subjecting to a prior predictive checking process
- Determining the likelihood function
- Combining the likelihood function with the prior distribution to form a posterior distribution
- Summarising resulting probabilities with associated point estimates
Markov Chain Monte Carlo (MCMC) based methods:
- Metropolis Hastings
- Reversible jump MCMC
- Hamiltonian Monte Carlo
- Gibbs Sampler
- Particles MCMC
- Evolutionary Monte Carlo
Other methods:
- Sequential Monte Carlo
- Approximate Bayesian Computation
- Integrated nested Laplace approximations
- Variational Bayes.
Advancing Clinical Trials with Biostatistical Methods: Bayesian Adaptive Designs and Master Protocols
In the realm of medical research and drug development, clinical trials play a pivotal role in assessing the safety and efficacy of new treatments. Biostatistics, a crucial discipline within the field of clinical research, offers innovative and adaptive approaches to optimise trial efficiency and enhance decision-making. Transformative biostatistical methods of Bayesian Adaptive Designs and Master Protocols are revolutionizing the landscape of clinical trials.
1. Understanding Bayesian Adaptive Designs
Bayesian Adaptive Designs are a powerful class of clinical trial designs that continuously update trial parameters based on accumulating data. Unlike traditional fixed designs, Bayesian methods allow for seamless incorporation of new information, making trials more efficient and responsive to real-time evidence.
1.1 Bayesian Inference in Clinical Trials
Bayesian inference is at the core of Bayesian Adaptive Designs. It allows researchers to combine prior knowledge (prior probabilities) and newly collected data (likelihood) to update beliefs about treatment effects (posterior probabilities). This iterative process helps researchers make data-driven decisions and adapt the trial as it progresses.
1.2 Advantages of Bayesian Adaptive Designs
The key advantages of Bayesian Adaptive Designs include:
- Efficient Sample Size: Bayesian methods often require smaller sample sizes than traditional designs to achieve the same level of statistical power, reducing costs and accelerating trial completion.
- Early Stopping: Bayesian designs enable early stopping for futility or efficacy, sparing patients unnecessary exposure to ineffective treatments.
- Seamless Adaptation: Researchers can adapt treatment arms, patient allocation ratios, or even eligibility criteria during the trial based on emerging data, making the trial more responsive to changing circumstances.
- Personalised Medicine: Bayesian methods facilitate subgroup analysis, allowing for the identification of patient populations that benefit the most from the treatment.
2. Master Protocols: Unifying Multiple Trials
Master Protocols, also known as umbrella, basket or platform trials, are innovative and adaptive trial designs that unify multiple sub-studies under a single infrastructure. These protocols streamline the evaluation of multiple treatments or interventions for a specific disease or condition.
2.1 Umbrella Trials
Umbrella trials group patients with a particular disease into different sub-studies based on specific biomarkers or genetic characteristics. Each sub-study evaluates a different targeted therapy or treatment, allowing for a personalized approach to patient care based on individual biomarker profiles.
2.2 Basket Trials
In contrast to umbrella trials, basket trials group patients with different diseases or conditions based on shared biomarkers or genetic alterations. This design allows for the evaluation of a specific targeted therapy or treatment across diverse patient populations, enabling efficient assessment of treatments for rare genetic abnormalities.
2.3 Platform Adaptation Trials
Platform Adaptation Trials are dynamic master protocols that continuously evolve to accommodate new treatments or interventions during the trial. They allow for the seamless addition or removal of treatment arms based on real-time data, promoting flexibility and efficiency in clinical research.
3. Biostatistical Considerations and Challenges
While Bayesian Adaptive Designs and Master Protocols offer numerous advantages, they also present unique biostatistical considerations and challenges.
3.1 Bayesian Sample Size Calculations
Bayesian sample size calculations require careful consideration of prior information, effect size assumptions, and the desired level of statistical power. Biostatisticians must strike a balance between updating prior beliefs and incorporating newly acquired data to ensure optimal trial efficiency.
3.2 Adaptive Randomization
Adaptive randomization methods need to be carefully designed to maintain trial integrity while accounting for potential biases and ethical considerations. Proper allocation mechanisms are essential to ensure unbiased treatment assignment and control potential confounding factors.
3.3 Data Monitoring and Interim Analyses
Biostatisticians play a crucial role in designing robust interim analysis plans to safeguard trial integrity. Continual data monitoring and adaptive decision-making require sound statistical methodologies to prevent unwarranted bias and maintain the validity of trial results.
Bayesian Adaptive Designs and Master Protocols represent cutting-edge biostatistical methods that revolutionise the landscape of clinical trials. These innovative approaches enable more efficient and responsive studies, ultimately accelerating medical research and improving patient outcomes. By incorporating Bayesian methods and unifying multiple trials under a master protocol, researchers can optimize trial efficiency, facilitate personalised medicine, and drive medical advancements towards a brighter future.
Survey design and development:
We ensure that a survey addresses your overarching research goals and evaluates the constructs it intends to measure by fine tuning every detail.
Development of your survey may involve the collection of qualitative responses (such as open-ended or free response style questions) or quantitative responses (such as nominal or Likert scale questions), or a degree of combining the two. Once the response style has been established we will assist in determining the right questions to include and how to ask them in order to optimise data collection in service of evaluating your constructs.
Sampling method determination:
This decision with be based on feasibility criteria in line with what best serves your research parameters such as distribution capabilities, budget, timeline and study questions. Randomised probability or stratified sampling, convenience sampling, or more targeted selective sampling can be used dependent on circumstances.
Online platform navigation:
We can help set up and implement your survey on the platform of your choice such as Qualtrics, survey monkey or Google Forms.
Pilot testing:
This involves running a pilot test of the survey and analysing results for reliability and construct validity using statistics such as test-retest reliability and Cronbach's alpha. From here any tweaks to the survey content can be made and confirmatory factor analysis can establish the statistical power of your final survey, which enables the appropriate sample size to be calculated.
Final survey analysis:
statistical analysis of your final survey data can involve factor analysis, principle components analysis, latent variable models, predictive models or many other options depending on the data and research questions involved.
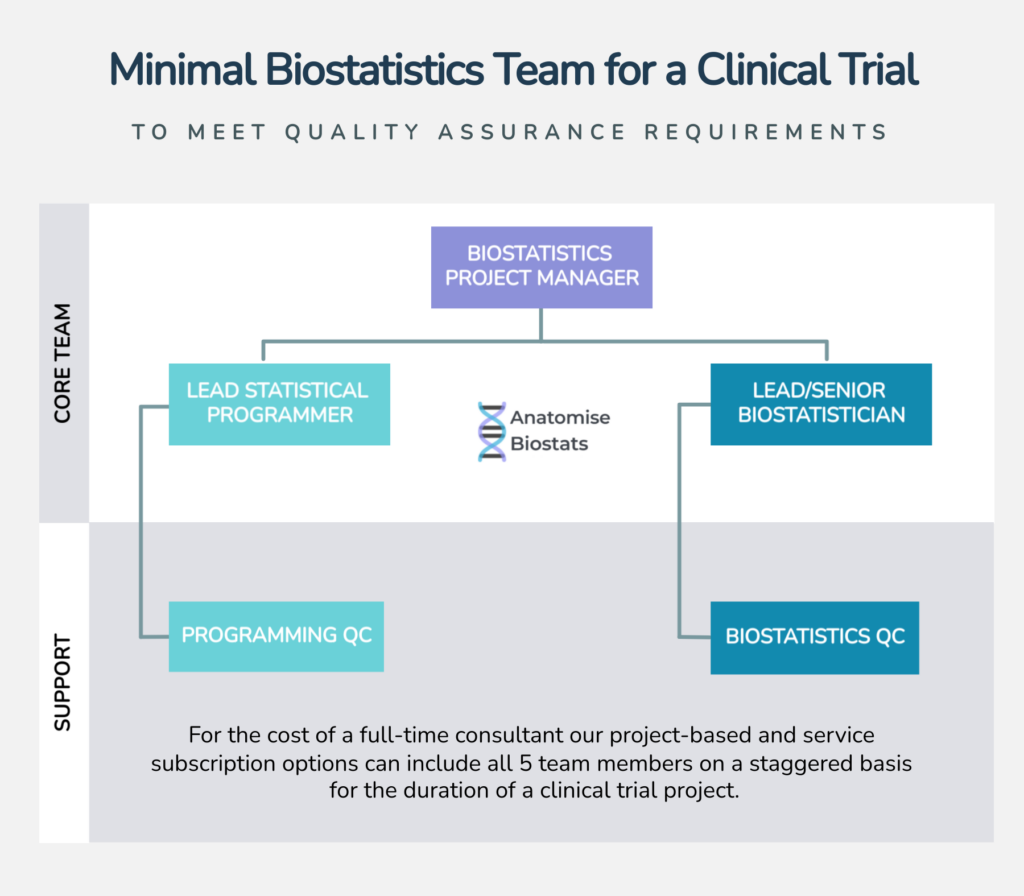
Statistical Programming for Clinical Trials
Statistical programmers play a crucial role in the execution and analysis of clinical trials. Their primary responsibilities involve translating study protocols and statistical analysis plans into executable programs that manage and analyse clinical trial data. This ensures that the data collected is accurately processed and that the resulting analyses are reliable and reproducible.
Key Responsibilities
- Data Management and Preparation
- Data Cleaning: Identifying and rectifying inconsistencies, missing values, and errors in the clinical trial data.
- Data Transformation: Converting raw data into standardised formats such as SDTM (Study Data Tabulation Model) and ADaM (Analysis Data Model) datasets to facilitate analysis.
- Database Setup: Creating and maintaining databases that store clinical trial data securely and efficiently.
- Programming Statistical Analyses
- Implementation of Statistical Analysis Plans (SAP): Translating the SAP into executable code to perform specified statistical tests and analyses.
- Creation of Programmable Datasets: Developing datasets that are ready for statistical analysis, ensuring they meet the requirements outlined in the SAP.
- Automating Processes: Developing scripts and macros to automate repetitive tasks, enhancing efficiency and reducing the potential for human error.
- Generation of Tables, Listings, and Figures (TLFs)
- Tables: Producing summary tables that display baseline characteristics, demographics, and treatment outcomes.
- Listings: Creating detailed listings of individual patient data and specific variables of interest.
- Figures: Developing graphical representations such as charts and graphs to visualise key findings and trends within the data.
Reporting and Documentation
- Statistical Reports: Compiling comprehensive reports that present the results of statistical analyses in a clear and structured manner.
- Traceability Matrices: Maintaining documentation that links each element of the SAP to the corresponding program outputs, ensuring transparency and traceability.
- Validation Documentation: Recording the validation processes undertaken to ensure the accuracy and reliability of the programming outputs.
Quality Assurance and Validation
- Code Validation: Testing and verifying programming code to ensure it accurately performs the intended analyses without errors.
- Data Validation: Conducting thorough reviews of datasets and outputs to confirm data integrity and compliance with regulatory standards.
- Compliance Checks: Ensuring all programming activities adhere to relevant regulatory guidelines, such as those set by the FDA and EMA.
Typical Deliverables:
Standardised Datasets
SDTM and ADaM datasets prepared according to regulatory standards for submission to regulatory authorities.
Statistical Analysis Programs
Executable code that performs the analyses outlined in the SAP, including descriptive statistics, hypothesis testing, and modeling.
Tables, Listings, and Figures (TLFs)
Comprehensive sets of TLFs that summarize and visualize the clinical trial data, facilitating the interpretation of results.
Statistical Reports
Detailed reports that present the findings of the statistical analyses, including methodologies, results, and interpretations.
Traceability Matrices
Documentation that ensures each aspect of the SAP is accounted for in the programming outputs, providing a clear audit trail.
Validation Documentation
Records of the validation processes carried out to verify the accuracy and reliability of the programming code and outputs.
Statistical programmers are integral to the successful execution of clinical trials, ensuring that data is accurately managed, analysed, and reported. Their expertise in programming and data handling facilitates the generation of reliable and compliant results, which are essential for the evaluation of clinical interventions and regulatory submissions.

We’ve got the details covered.
We help you to develop and implement a clear plan of action from the outset of your project to ensure you remain on your optimal trajectory for the project duration.
In the initial stages we refine your endpoints to maximally address your research questions and develop a statistical analysis plan (SAP) that will optimise your study results.
Study power and sample size vary wildly as a function of statistical test and study design. Defining the SAP early is key to accurate the sample size and power estimation.
We advise on eCRF, data collection and inputting techniques that reduce the need for data cleaning and preparation at the analysis stage, whether STDM and ADaM for clinical trials or the collation of RWD for modelling purposes.
We fine tune your SAP, if needed, to align with any unexpected developments. Upon final analysis study results are presented clearly, with straightforward guidance on interpretation to support your next steps.
